Which of the Following Exists as a Polyatomic Molecule
Hence the correct option is c. Oxygen exists as O2.

Solved 51 In Which Set Do All Elements Tend To Form Anions Chegg Com
For example oxygen can exist as the triatomic molecule ozone.

. The elements which exist as groups of more than two atoms are called polyatomic elements. The science of molecules is called molecular chemistry or molecular physics depending on the focus. The noble gases are monatomic gases.
Molecular Chemistry and Molecular Physics. Are polyatomic elements. Which of the following exists as a polyatomic molecule.
DrBob222 Apr 8 2012 Respond to this Question Similar Questions CHEM. Ionic compounds is composed of a metal and nonmetal. If the mass of an oxygen atom is 266 x kg and the observed frequency of oscillation is Hz what is the effective spring constant associated with the bond between the.
H-H Cl-Cl etc are examples of the latter. Oxygen bPhosphorus cSulphur dArgon d Argon Oxygen is diatomic Phosphorus is tetra-atomic Sulphur is poly-atomic and Argon is monoatomic. WHICH OF THE FOLLOWING EXISTS AS A POLYATOMIC MOLECULE.
The atoms in all substances that contain multiple atoms are held together by electrostatic interactionsinteractions between electrically charged pwrite-ups such as proloads and also electrons. Electrostatic attractivity between oppositely charged species positive and negative. Additional Information Any molecule that contains more than 2 atoms is polyatomic.
Sulphur is a polyatomic molecule as it exists in nature as S6 and S8. Types of molecules Monoatomic molecules. Ate ending in a.
Information about various chemical compounds and elements. Write the name for SnSO42 Tin IV Sulfate Which of the following exists as a polyatomic molecule. Which of the following elements exists as a diatomic molecule A neon B lithium C from BIO 3 at University of Caloocan City formerly Caloocan City Polytechnic College.
A number of elements are found in their elemental form as diatomic moleculesIn these molecules two atoms are joined by one or more covalent bonds forming a molecule with the general formula X 2. And phosphorous P 4. The elements that have only one atom in the molecule are called monoatomic elements.
The number can be calculated from the following. One atom in the molecule can move independently in three directions the x y and z directions in a Cartesian coordinate system. Question Which of the following is an example of polyatomic molecule.
Molecules are distinguished from ions by their lack of electrical charge. Carbon phosphorus argon sodium neon. Polyatomic ions consist of ions that have charges that cancel each other.
The correct answer is Sulfur. Argon and helium are noble gases or are monoatomic elements. The elements that have only one atom in a molecule are called monoatomic.
All the others are polyatomic molecules. Lithium neon carbon phosphorus krypton. This is called atomicity.
Correct options are A and B Sulphur S 8. A molecule can also have even more than 3 covalently bonded atoms. Is selenium metal toxic.
Hence Sulfur is polyatomic. Polyatomic molecules have more than one vibrational frequency. Suggest corrections 0 Upvotes Similar questions.
Diatomic molecules such as and undergo simple harmonic motion with frequencies that obey Hookes Law with the effective mass being half the atomic mass. Hence option 3 is correct. These elements can exist in pure form in other arrangements.
Click to see full answer. There are only three polyatomic elements in the periodic table namely- selenium sulphur and phosphorous. Maci Apr 8 2012 It must be symmetrical or it must hava elements with the same electronegativity.
The elements found as diatomic molecules are hydrogen H element 1 nitrogen N element 7 oxygen O element. Hydrogen nitrogen oxygen fluorine chlorine iodine bromine. Polyatomic molecules are electrically neutral groups of three or more atoms held together by covalent bonds.
Chlorine Cl 2 Nitrogen N 2 and Hydrogen H 2 are diatomic gases. Polyatomic molecule synonyms Polyatomic molecule pronunciation Polyatomic molecule translation English. Any molecule that contains more than 2 atoms is polyatomic.
Molecular compounds is composed of two or more covalently bonded non-metals. CH4 and CO2 are examples of the former. But sulphur exists as S8 and hence it is polyatomic.
Nitrogen exist as diatomic molecule. Therefore it exists in its elemental form as He. Which of the following elements exists as a diatomic molecule A neon B lithium C.
The molecules of elements can be monoatomic diatomic triatomic and so on depending on the number of atoms in each molecule of the element. There are seven diatomic elements. It appears in diatomic form as O_2 which is a combination of two oxygen atoms.
Selenium Se 8 Ozone O 3 and Phosphorous P 4 some other polyatomic elements. Key Points Sulfur is polyatomic. These molecules are commonly termed as polyatomic molecules or radicals.
Which of the following exists as a polyatomic molecule. Nitrogen Hydrogen Sulphur Oxygen Solution The correct option is C Sulphur Polyatomic molecules are groups of three or more atoms held together by covalent bonds. Colthup in Encyclopedia of Physical Science and Technology Third Edition 2003 ID Polyatomic Vibrations.
Elemental selenium has low toxicity following oral administration. A polyatomic element is a chemical element that naturally exists as a compound molecule containing more than three atoms of the same element. See the answer See the answer done loading.
Sulfur exists as S8 Hydrogen exists as H2. What conditions must exist for a polyatomic molecule to be nonpolar.
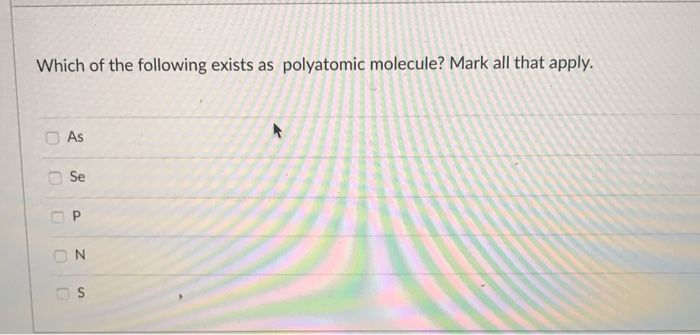
Solved Which Of The Following Exists As Polyatomic Molecule Chegg Com
Chapter Exercises Chemical Bonds I Ppt Download

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
No comments for "Which of the Following Exists as a Polyatomic Molecule"
Post a Comment